Van Der Waals Equation
Van Der Waals Equation: Overview
This topic covers concepts such as Van der Waals Equation for Real Gases, Units of Van der Waals Constants, Volume Correction for Real Gases, Factors Responsible for Volume Corrections, Pressure Correction for Real Gas, Van der Waals Constants, etc.
Important Questions on Van Der Waals Equation
In Van der Waal’s equation of state for a non-ideal gas, the term that accounts for intermolecular forces is:
When deviation is more in the behaviour of a gas from the ideal gas equation PV = nRT ?
Among the following, Van der Waals constant would be maximum for _____
The van der Waals coefficient (expressed in ) for four different gases are: Based on the data given above, the gas that will be expected to have the lowest critical temperature
Which of the following expressions is correct between the van der Waals constant and the radius of spherical molecules?
The Van der Waal's equation for real gases is
In the above equation, the terms a and , respectively represents the corrections for:
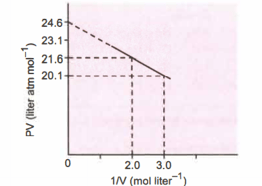
For one mole of a van der Waals gas when and , the plot is shown below. The value of the van der Waals constant is
mole of real gas occupies a volume of at , the pressure of gas will be:
The correction factor to the ideal gas equation corresponds to
If is the volume of one molecule of a gas, the van der waal's constant is:
For a real gas under low pressure conditions, which of the following graph is correct?
In Van der Waal equation at constant temperature what is the pressure of gas:
In the Vander Waal's equation, signifies:
For real gases, Van der Waals equation is written as.
where and are Van der Waals constants.
Two sets of gases are:
and
and
The gases given in set- in increasing order of and gases given in set- in decreasing order of , are arranged below. Select the correct order from the following:
At high temperature and low pressure van der Waal's equation can be expressed as
Vander Waal's constants 'a' and 'b' are related with....., respectively.
The correction factor '' to the ideal gas equation corresponds to?
For the corrections made to ideal gas equation to have real gases, identify the pressure reduction due to forces of attractions between the molecules is directly proportional to :
The Van der Waal's equation of state for mole is
Report Boyle's Temperature .
The correct form of van der Waals' equation at low pressures is:
